Have you ever noticed how easy it is for your bedroom to become messy, but how much work it takes to keep it perfectly clean? That natural drift from order to chaos is the essence of Entropy.
Entropy is one of the most fundamental concepts in science and a critical component of General Systems Theory (GST). It is a universal law, also known as the Second Law of Thermodynamics. This law states that every system in the universe naturally moves toward a state of increasing disorder, randomness, and decay. Understanding entropy is essential because it explains why life—and every organized system—must constantly work to survive.
The Universal Rule of Disorder
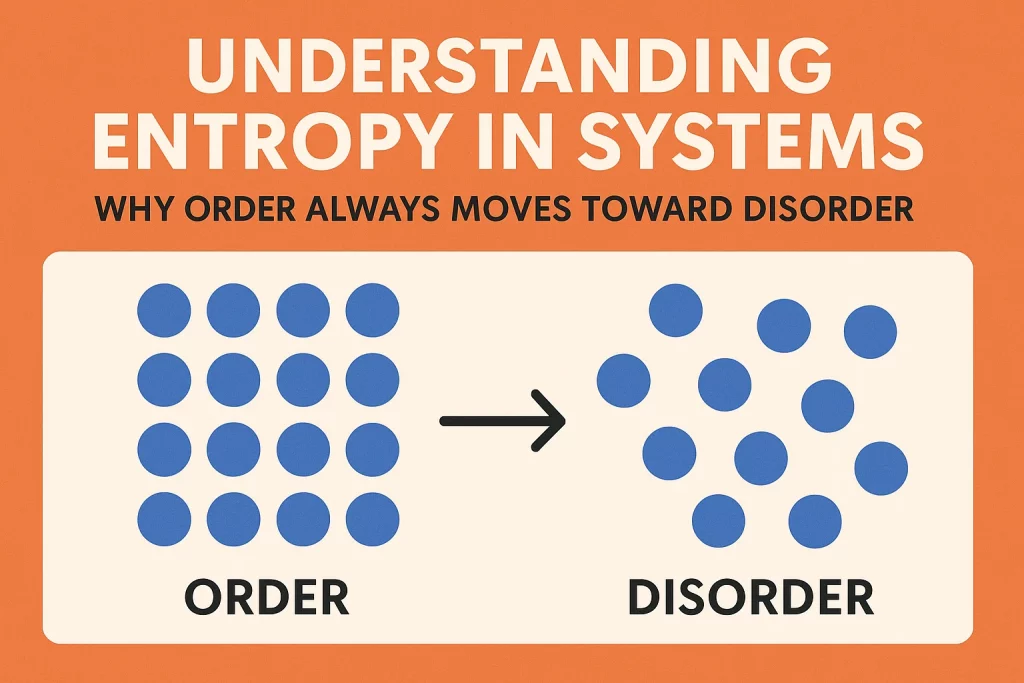
In simple terms, entropy is the measure of the disorder or randomness in a system. The more disordered a system is, the higher its entropy.
Energy is Always Lost
The law of entropy is directly related to energy. When energy is used, some of it is always “lost” or converted into a less useful form, usually heat that disperses into the environment. This means that every time a system does work—whether it’s an engine running or a human thinking—it becomes less organized.
- Closed Systems: In a closed system (one that doesn’t exchange matter, like a sealed battery), entropy must always increase. The battery runs down, the components wear out, and the system eventually reaches a state of complete, unusable equilibrium.
- The Arrow of Time: Entropy defines the direction of time. Since disorder (high entropy) can never spontaneously reverse itself back into perfect order (low entropy), entropy is what makes time move forward.
The Tendency Toward Uniformity
Entropy is also the tendency for differences to even out. For example, if you drop a dye into a glass of water, the dye will eventually spread until the color is uniform. The organized state (concentrated dye) naturally becomes a disorganized state (spread-out dye). The highly organized state is much less likely to happen than the disorganized state.
Entropy is the measure of disorder in a system and is governed by the Second Law of Thermodynamics. It dictates that all systems naturally tend toward maximum disorder, randomness, and decay, requiring constant effort and energy input to maintain organization.
How Open Systems Fight Entropy
If entropy is a universal law, how can living things, which are highly organized and complex, exist at all? This is where the GST concept of the Open System comes in.
Importing Low Entropy
Open Systems (like a plant, an animal, or a company) fight entropy by continually taking in matter and energy from their environment. They take in organized, low-entropy resources (like sunlight, food, or raw materials) and use that energy to rebuild their internal structures.
- The Exchange: The open system maintains its internal order by taking in low-entropy energy and exporting high-entropy waste (like heat and waste products) back out to the environment.
- Local Order: The system is only able to create local areas of order (like a clean room or a healthy body) at the expense of increasing the total entropy of the universe outside its boundary.
The Cost of Order
Every organized system exists because it is constantly working to repair, replace, and maintain its structure against entropic decay. This work requires a continuous input of energy.
- Biological Example: Your body is constantly fighting entropy by repairing cells and regulating temperature, a process that requires food (low-entropy energy).
- Organizational Example: A company must constantly invest energy (time, money, labor) in training, maintenance, and planning to keep its structure and processes from becoming chaotic and falling apart.
Conclusion
Entropy is the universal law of disorder, proving that the natural tendency of all systems is to decay and run down. This core principle of science and GST explains why work and energy flow are necessary for all systems to survive. By adopting the Open System structure and continually importing low-entropy resources, organized systems temporarily resist disorder, proving that life itself is a continuous, active battle against the pull toward chaos.